How Many Valence Electrons Does Bismuth Have
Therefore its a f-block element. Therefore the valence electrons of bismuth are five.
Valence Electrons In Beryllium Study Guide Inspirit
Determine the number of electrons in the Cr3 ion.

. Oxygen is in group 6 and has 6 valence electrons. Number of valence electrons 5 Express your answer as a series of orbitals in order of. But for most of the.
They are nitrogen phosphorus arsenic antimony and bismuth. To know these properties of einsteinium. Naturally Occuring Isotopes Typical Unstable Isotopes Electrons and Electron Configuration The number of.
In this way by knowing the position of bismuth element in periodic table you can easily find its. Bismuth has 5 valence electrons. Ie all group 1 elements have 1 valence electron all group 2.
There are two types of bismuth ion. The charge of electrons is 1609 10 19 coulombs and the relative charge is 1. Now the atomic number of Bismuth Bi is 83.
Boujieemyaa boujieemyaa 03092021 Chemistry High School answered How many Valance electrons does. The last electron of einsteinium enters the f-orbital. So as the bismuth element is present in group 15 it has 5 valence electrons.
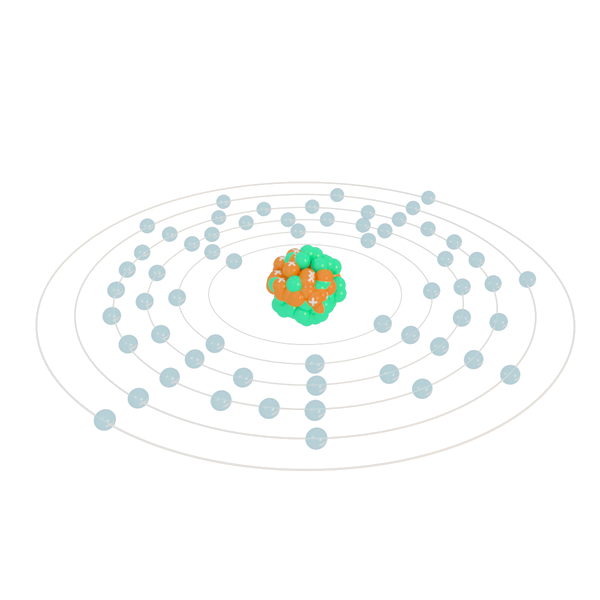
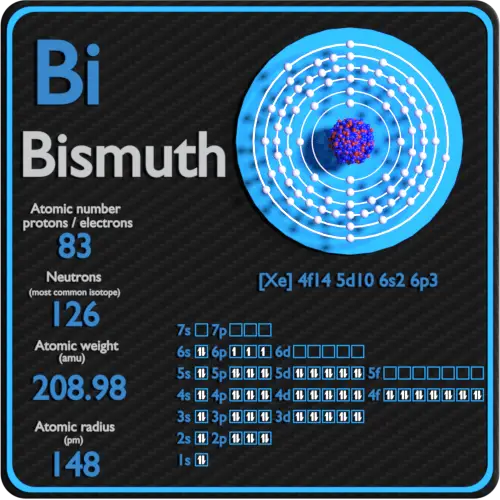
Bismuth has a valence electron count of. Bismuth atoms have 83 electrons and the electronic shell structure is 2 8 18 32 18 5 with Atomic Term Symbol Quantum Numbers 4S32. To know these properties of bromine one must know.
Ok but how many valence electrons does an atom of Bismuth have. Bismuth contains 5 electrons in its valence shell or its outer shell thus it contains 5 valence electrons. Its atomic number is 83.
Therefore the valence electrons of bromine are seven. We must also remember. For example carbon is in group 4 and has 4 valence electrons.
Therefore the valence electrons of bismuth are five. In the case of Bismuth the valence electrons is 35. For main group elements ie s-block and p-block elements the valence electrons are the electrons present in the outermost orbit.
It is a silvery-white metal that occurs naturally as bismuthinite Bi4SbO6 and bismuthinite- Ce. The last electron of bromine enters the p-orbital. Nitrogen Phosphorus Arsenic Antimony and Bismuth all have 5 valence electrons How many valence.
Bismuth has 5 valence electrons. Its atomic mass is 207904. Bismuth atoms have 83 electrons and the.
The valence electrons of each main group element can be determined by the column in which it is located. Express your answer in condensed form. What electrons are in bismuth.
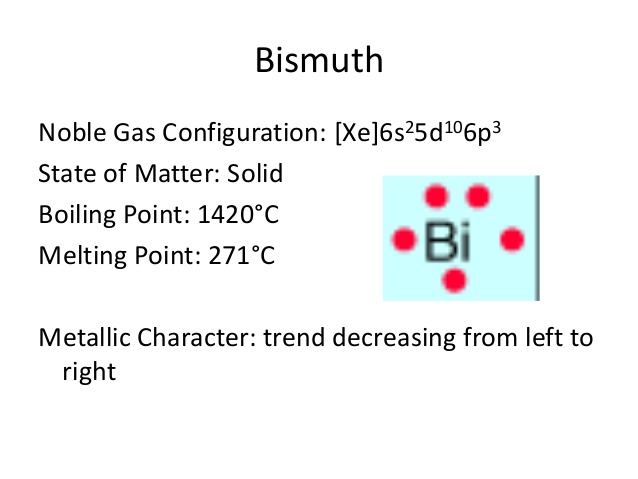
The elements that form bonds by donating. Use the periodic table to determine the electron configuration of bismuth Bi. The bismuth atom exhibits Bi 3 and Bi 5 ions.
Antimony is used in alloys with other metals to make them harder. The last electron of bismuth. How many Valance electrons does Bismuth have.
Bismuth-210 is composed of 83 protons 127 neutrons an 83 electrons. This electron arrangement indicates that the. Now the electron configuration of bismuth shows that the last shell of bismuth has five electrons.
As Bismuth is a Pentavalent post-transition metal thus it can be noted that it. Therefore the valence electrons of einsteinium are thirteen. That is the charge of an electron is equal to that of a proton but the opposite.
Atoms have 5 valence electrons. How many valence electrons does Bi have and what are the specific valence electrons for Bi. Therefore its a p-block element.
It is also used. Hence the Bismuth element has electrons arrangement 2 8 18 32 18 5. Now lets check the facts about Bismuth.

The Parts Of The Periodic Table

Chemistry The Central Science Chapter 6 Section 9

A The Calculated Valence Electron Densities Of The Clean Bismuth 001 Download Scientific Diagram

Happy Halogens Predicting The Number Of Valence Electrons
Chemical Elements Com Bismuth Bi

Chemistry The Central Science Chapter 6 Section 9

Electronic Configuration Group 15 Elements List First 30 Elements
Bismuth Protons Neutrons Electrons Electron Configuration

Bismuth Properties Uses Symbol Facts Britannica

Bismuth As A Reactive Solvent In The Synthesis Of Multicomponent Transition Metal Bearing Bismuthides Inorganic Chemistry

How Many Valence Electrons Do Each Of The Following Elements Quizlet

Solved The Lewis Dot Structure Of An Atom Of Bismuth Bi Will Have 2 Electron Pairs And 1 Unpaired Electron 5 Unpaired Electrons 1 Electron Pair And 3 Unpaired Electrons 1 Electron Pair And Three Electrons Together
Where To Find A Electron Configuration For Bismuth Bi

Solved Valence Electrons Are Defined As Electrons In The Chegg Com

Mecchapter2 120815075639 Phpapp02

Bismuth Wikipedia

Rcsb Pdb Bs3 Ligand Summary Page